Participating in a clinical trial can be an exciting and important step in advancing medical research, as well as potentially improving your health condition. If you are considering joining a clinical trial, it is essential to understand what the process involves and what you can expect throughout your participation. In this blog post, we will walk you through the steps involved in a clinical trial, from the initial screening to the end of the study, and what to expect at each stage.
1. Screening and Enrollment
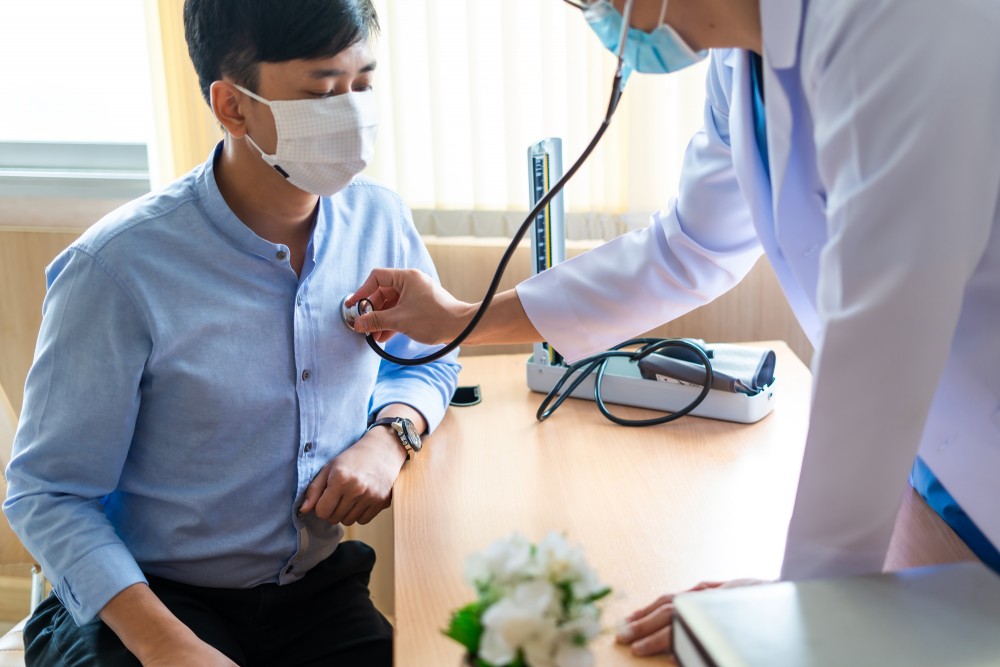
The first step in participating in a clinical trial is the screening process. During this phase, researchers will assess your eligibility to determine if the trial is appropriate for you. This may involve answering questions about your medical history, undergoing physical exams, and providing samples such as blood or urine. The purpose of the screening is to ensure that you meet the necessary criteria and that the trial will be safe for you to participate in.
If you are eligible, you will be provided with all the information you need to make an informed decision, including a detailed consent form outlining the purpose of the study, potential risks, benefits, and your rights as a participant. Once you fully understand the trial details and agree to participate, you will be officially enrolled.
2. During the Trial: Monitoring and Treatment
Once enrolled, you will begin the actual treatment phase of the clinical trial. Depending on the study design, you may receive the experimental treatment or therapy being tested, a placebo, or the standard treatment. Throughout the trial, you will have regular visits with the research team to monitor your health and ensure the study is progressing as expected.
These visits may include physical exams, lab tests, and assessments to track your progress and any side effects you may experience. You will be closely monitored to ensure that the treatment is effective and that you are not experiencing any adverse reactions. The research team will provide ongoing support and will be available to answer any questions or concerns you may have during the trial.
3. Understanding the Risks and Benefits
It is important to be aware of both the potential risks and benefits before joining a clinical trial. While clinical trials aim to provide new treatments that can be beneficial, there may also be risks involved, such as side effects or complications. The research team will explain the possible risks in detail and discuss how they will minimize these risks to protect your health.
On the other hand, participating in a clinical trial may offer access to cutting-edge treatments that are not yet available to the general public. Additionally, your participation helps advance scientific knowledge and can potentially contribute to the development of treatments that will benefit future patients. Throughout the trial, you will have the option to withdraw at any time if you feel uncomfortable or if the trial no longer meets your needs.
4. End of the Trial and Follow-up
At the end of the clinical trial, you will typically have a final visit with the research team to assess the results and ensure your well-being. Depending on the study, you may receive feedback on the trial’s findings, although the specifics may not be available until the study is completed and the data is analyzed.
Even after the trial ends, you may be invited to participate in follow-up visits or post-trial monitoring to ensure that any long-term effects of the treatment are noted. The follow-up phase is an important part of the trial, as it helps researchers gather additional data on how the treatment affects participants in the long term.
5. What You Can Expect from the Clinical Trial Team
Throughout your participation in the clinical trial, the research team will be there to support you. The team typically includes doctors, nurses, researchers, and other healthcare professionals who are dedicated to your safety and well-being. They will guide you through each phase of the trial and provide you with any information you need to understand the process.
Your health and comfort are the primary concerns for the clinical trial team. If you experience any discomfort, side effects, or changes in your health, the team will work with you to address those issues. They will also be available to answer any questions you may have about the treatment, trial procedures, or your health status.
6. Compensation and Reimbursement
In some clinical trials, participants may be compensated for their time and travel expenses. Compensation varies depending on the trial and may include reimbursement for transportation, parking, or other related costs. It’s important to clarify the details of compensation with the research team before enrolling in a trial to understand what, if any, compensation will be provided.
Conclusion
Participating in a clinical trial can be a life-changing experience, offering potential benefits for both your health and the broader medical community. By understanding the steps involved and what to expect throughout the process, you can make an informed decision about whether a clinical trial is right for you. From screening and enrollment to treatment, monitoring, and follow-up, the research team will guide you every step of the way. If you have any concerns or questions along the way, they are there to support you. Your participation could contribute to vital advancements in healthcare and may offer access to new treatments that improve your health.